Kv5.1
Description: potassium voltage-gated channel, subfamily F, member 1 Gene: Kcnf1 Alias: Kv5.1, kcnf1, kh1, ik8, kcnf
Kv5.1 (also known as K8; kH1; KCNF; MGC33316), encoded by the gene KCNF1, is a member of the voltage-gated, subfamily F. Kv5.1 channels cannot generate K+ channel activity by themselves, but modulate in a specific way the function of Kv2.1 and Kv2.2 subunits [400], [399]. NCBI
Experimental data
Rat Kv5.1 gene in CHO host cells datasheet |
||
|
Click for details 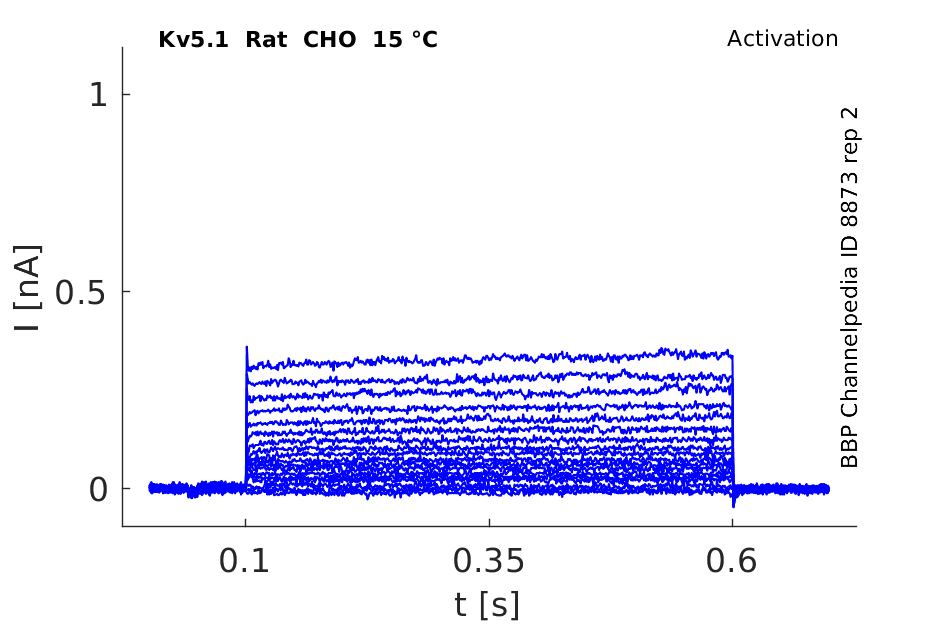
15 °Cshow 50 cells |
Click for details 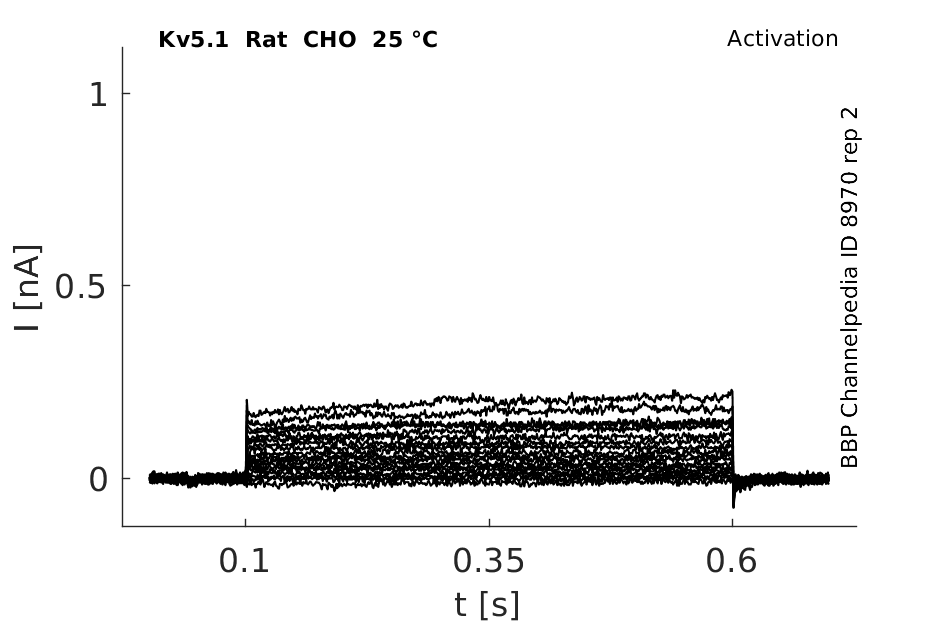
25 °Cshow 58 cells |
Click for details 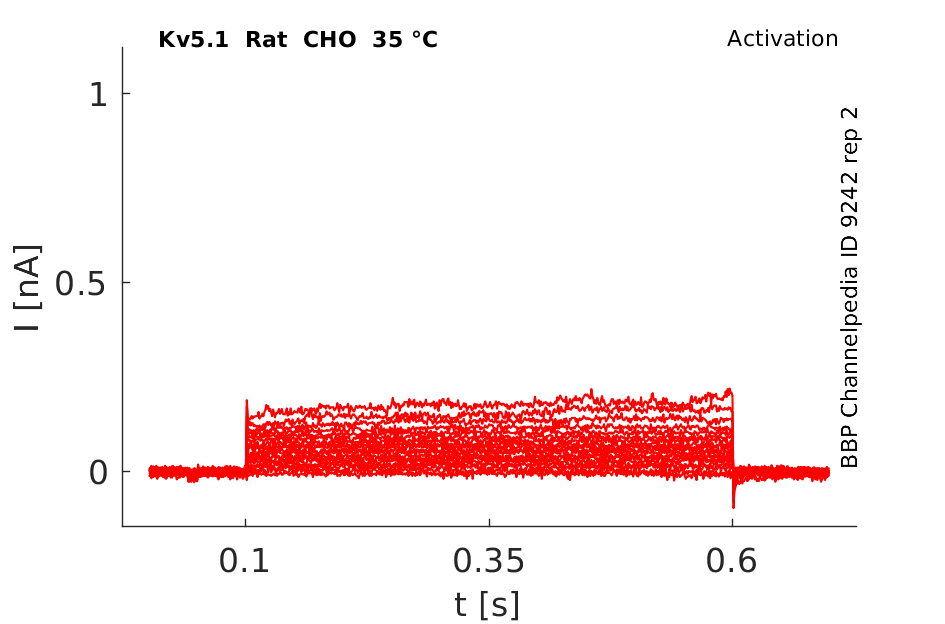
35 °Cshow 61 cells |
KCNF1 is intronless and expressed in all tissues tested, including the heart, skeletal muscle, brain, kidney, and pancreas. NCBI
Transcript
| Species | NCBI accession | Length (nt) | |
|---|---|---|---|
| Human | NM_002236.5 | 2292 | |
| Mouse | NM_201531.4 | 4789 | |
| Rat | NM_001169104.1 | 2679 |
Protein Isoforms
Isoforms
Post-Translational Modifications
Visual Representation of Kv5.1 Structure
Methodology for visual representation of structure available here
STRUCTURE AND COMPARISON OF HUMAN AND RAT KV5.1
kH1 and Kv5.1 (IK8) are completely identical in S3, S4, S5, S6 and H5 domains. This probably underlines the importance of the core region for K/ channel function and it associated properties. At the N-terminal tail, IK8 is 12 amino acid longer than kH1. At the beginning of 5'end, kH1 is followed by a 6 amino acid sequence which is mismatched. At the C-terminal cytoplasmic tail, kH1 and IK8 are distinguished by one gap and ten amino acids which are mismatched [1698]
Kv5.1 predicted AlphaFold size
Methodology for AlphaFold size prediction and disclaimer are available here
Kinetic interaction of Kv5.1 and Kv2
Kv5 and Kv6 are members of the electrically silent families that are capable of evoking a large negative shift in the steady-state inactivation of Kv2.1- and Kv2.2-containing channels. The half-maximal steady-state inactivation values reported for these heteromultimeric channels are consistent with the value of −61 mV reported here for the UBSM IK(V). Steady-state activation of the UBSM IK(V) demonstrates a half-maximal value of 1.1 mV, which is mid-range of that reported for Kv2.1 (between 10 mV and −1.7 mV), is more negative than Kv2.1/Kv5.1 (18 mV) and is more positive than Kv2.1/Kv6.1 (-9.4 mV). It seems possible that the half-maximal steady-state activation value of a given heteromultimeric channel will be dependent on the stoichiometry of Kv2.1, Kv5.1 and Kv6.1 channel subunits [1699]
Expression of Kv5.1
kH1 was expressed abundantly in tissues examined, including the heart, skeletal muscle, and less abundant in the brain, liver, kidney, and pancreas [1698]
Northern blot analysis revealed that KH1 was expressed as a 5-kb mRNA in all tissues tested, with the highest levels in heart. A 2.4-kb transcript was detected only in brain (omim.org)
UBSM and mouse brain tissue
T-PCR for voltage-gated K+ channel (KV) subunits revealed the expression of Kv2.1, Kv5.1, Kv6.1, Kv6.2 and Kv6.3 in isolated urinary bladder smooth muscle myocytes as well as mouse brain. A comparison of the biophysical properties of UBSM IK(V) with those reported for Kv2.1 and Kv5.1 and/or Kv6 heteromultimeric channels demonstrated a marked similarity. We propose that heteromultimeric channel complexes composed of Kv2.1 and Kv5.1 and/or Kv6 subunits form the molecular basis of the mouse UBSM IK(V) [1699]
KV5.1 has no function on its own, but has important modulatory actions on KV2 channels [399]
Kv2.1
Kv5.1 coexpression slowed deactivation of Kv2.1, accelerated the rate of inactivation of Kv2.1 at intermediate potentials (-30 to 0 mV), without affecting the rate at strong depolarization (0 to 40 mV), and markedly accelerated the rate of cumulative inactivation evoked by high-frequency trains of short pulses.[400], [398]
The effects of coexpression of Kv2.1 with electrically silent Kv5.1 or Kv6.1 alpha-subunits in Xenopus oocytes on channel gating. Kv5.1 coexpression alsoslowed deactivation of Kv2.1. In contrast, Kv6.1 was much less effective in speeding inactivation at intermediate potentials, had a slowing effect on inactivation at strong depolarizations, and had no effect on cumulative inactivation
References
Rettig J
et al.
Inactivation properties of voltage-gated K+ channels altered by presence of beta-subunit.
Nature,
1994
May
26
, 369 (289-94).
Kramer JW
et al.
Modulation of potassium channel gating by coexpression of Kv2.1 with regulatory Kv5.1 or Kv6.1 alpha-subunits.
Am. J. Physiol.,
1998
Jun
, 274 (C1501-10).
Post MA
et al.
Kv2.1 and electrically silent Kv6.1 potassium channel subunits combine and express a novel current.
FEBS Lett.,
1996
Dec
9
, 399 (177-82).
Salinas M
et al.
New modulatory alpha subunits for mammalian Shab K+ channels.
J. Biol. Chem.,
1997
Sep
26
, 272 (24371-9).
Strausberg RL
et al.
Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences.
Proc. Natl. Acad. Sci. U.S.A.,
2002
Dec
24
, 99 (16899-903).
Ottschytsch N
et al.
Obligatory heterotetramerization of three previously uncharacterized Kv channel alpha-subunits identified in the human genome.
Proc. Natl. Acad. Sci. U.S.A.,
2002
Jun
11
, 99 (7986-91).
Isacoff EY
et al.
Evidence for the formation of heteromultimeric potassium channels in Xenopus oocytes.
Nature,
1990
Jun
7
, 345 (530-4).
Pongs O
Molecular biology of voltage-dependent potassium channels.
Physiol. Rev.,
1992
Oct
, 72 (S69-88).
Su K
et al.
Isolation, characterization, and mapping of two human potassium channels.
Biochem. Biophys. Res. Commun.,
1997
Dec
29
, 241 (675-81).
Thorneloe KS
et al.
Properties and molecular basis of the mouse urinary bladder voltage-gated K+ current.
J. Physiol. (Lond.),
2003
May
15
, 549 (65-74).
Contributors: Rajnish Ranjan, Michael Schartner, Nitin Khanna
To cite this page: [Contributors] Channelpedia https://channelpedia.epfl.ch/wikipages/18/ , accessed on 2024 May 04