Cav3.3
Description: calcium channel, voltage-dependent, T type, alpha 1I subunit Gene: Cacna1i Alias: cacna1i, cav3.3, ca3.3, Ca(v)3.3
CACNA1I (also known as Cav3.3; KIAA1120) encodes Cav3.3, a T type LVA calcium channel found in neurons which is also know as a1I. Voltage-dependent calcium channels control the rapid entry of Ca(2+) into a variety of cell types and are therefore involved in both electrical and cellular signaling. T-type channels, such as CACNA1I, are activated by small membrane depolarizations and can generate burst firing and pacemaker activity
http://www.ncbi.nlm.nih.gov/gene/8911
Gene
Transcript
| Species | NCBI accession | Length (nt) | |
|---|---|---|---|
| Human | NM_021096.4 | 10002 | |
| Mouse | NM_001044308.2 | 9833 | |
| Rat | NM_020084.3 | 6709 |
Protein Isoforms
Isoforms
Post-Translational Modifications
Structure
Cav3.3 predicted AlphaFold size
Methodology for AlphaFold size prediction and disclaimer are available here
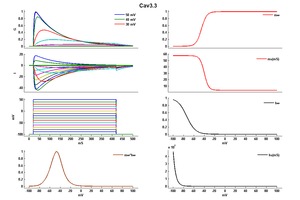
T-type channels are distinguished from high voltage-activated (HVA)1 Ca2+ channels by their unique biophysical properties, including low voltage activation, fast activation and inactivation kinetics that produce a criss-crossing pattern between successive traces of a current-voltage (IV) protocol, slow deactivation kinetics, and tiny single channel conductance (Perez-Reyes [528], Armstrong [1237], Carbone [1238], Randall [340]).
Expression studies found that Cav3.3 channels generate currents with much slower activation and inactivation kinetics than Cav3.1 and Cav3.2 channels, which show the more typical transient kinetics described for native T-type channels (Perez-Reyes [528], Perez-Reyes [1239], Cribbs [1240], Lee [1241]).
Cav3.1 and Cav3.2 channels are activating and inactivating much faster than Cav3.3 channels. (Park [113])
The kinetics of T-type channels resemble those of Na+ channels, albeit on a slower time scale, suggesting that they may also inactivate by a ball-and-chain mechanism. However, preliminary evidence indicates that T-type channels inactivate by similar processes as HVA Ca2+ channels.Multiple structural elements contribute to the slow kinetics of Cav3.3 channels. (Park [113])
Biophysics
Model Cav3.3 (ID=42)
| Animal | CH | |
| CellType | CHO | |
| Age | 0 Days | |
| Temperature | 0.0°C | |
| Reversal | 30.0 mV | |
| Ion | Ca + | |
| Ligand ion | ||
| Reference | [103] Achraf Traboulsie et. al; J. Physiol. (Lond.) 2007 Jan 1 | |
| mpower | 1.0 | |
| m Inf | 1/(1+exp((v- -45.454426)/-5.073015)) | |
| m Tau | 3.394938 +( 54.187616 / (1 + exp((v - -40.040397)/4.110392))) | |
| hpower | 1.0 | |
| h Inf | 1 /(1+exp((v-(-74.031965))/8.416382)) | |
| h Tau | 109.701136 + (0.003816 * exp(-v/4.781719)) | |
T-type Ca2+ currents are central determinants of neuronal excitability that are present in the somatodendritic compartment of many types of neurones (Carbone & Lux, 1984 [1238]; Talley et al. 1999 [1242]).
Genetic and pharmacological inhibition of T-type Ca2+ currents has demonstrated the importance of these currents in various sensory systems, ranging from pain perception and hyperalgesia (Kim et al. 2003 [1243]; Ikeda et al. 2003 [1244]), mechanoreceptor function (Shin et al. 2003 [1245]) and to olfaction (Kawai & Miyachi, 2001 [1246]).
Zinc
CaV 3.2 current (IC50 , 0.8 μM) is significantly more sensitive to Zn2 + than are CaV 3.1 and CaV 3.3 currents (IC50 , 80 μM and ∼160 μM, respectively). This inhibition of CaV 3 currents is associated with a shift to more negative membrane potentials of both steady-state inactivation for CaV 3.1, CaV 3.2 and CaV 3.3 and steady-state activation for CaV 3.1 and CaV 3.3 currents. We also document changes in kinetics, especially a significant slowing of the inactivation kinetics for CaV 3.1 and CaV 3.3, but not for CaV 3.2 currents. (Traboulsie [103])
Phorbol-12-myristate-13-acetate (PMA)
PMA augmented the current amplitudes of the three T-type channel isoforms (Cav3.1, Cav3.2, Cav3.3), but the fold stimulations and time courses differed. (Park [112])
References
Traboulsie A
et al.
Subunit-specific modulation of T-type calcium channels by zinc.
J. Physiol. (Lond.),
2007
Jan
1
, 578 (159-71).
Park JY
et al.
Activation of protein kinase C augments T-type Ca2+ channel activity without changing channel surface density.
J. Physiol. (Lond.),
2006
Dec
1
, 577 (513-23).
Park JY
et al.
Multiple structural elements contribute to the slow kinetics of the Cav3.3 T-type channel.
J. Biol. Chem.,
2004
May
21
, 279 (21707-13).
T-type Ca2+ channels encode prior neuronal activity as modulated recovery rates.
J. Physiol. (Lond.), 2006 Mar 15 , 571 (519-36).
Cataldi M
et al.
Zn(2+) slows down Ca(V)3.3 gating kinetics: implications for thalamocortical activity.
J. Neurophysiol.,
2007
Oct
, 98 (2274-84).
Kovacs K
et al.
Subcellular distribution of low-voltage activated T-type Ca2+ channel subunits (Ca(v)3.1 and Ca(v)3.3) in reticular thalamic neurons of the cat.
J. Neurosci. Res.,
2010
Feb
1
, 88 (448-60).
Randall AD
et al.
Contrasting biophysical and pharmacological properties of T-type and R-type calcium channels.
Neuropharmacology,
1997
Jul
, 36 (879-93).
Perez-Reyes E
Molecular physiology of low-voltage-activated t-type calcium channels.
Physiol. Rev.,
2003
Jan
, 83 (117-61).
Armstrong CM
et al.
Two distinct populations of calcium channels in a clonal line of pituitary cells.
Science,
1985
Jan
4
, 227 (65-7).
Carbone E
et al.
A low voltage-activated, fully inactivating Ca channel in vertebrate sensory neurones.
Nature,
1984 Aug 9-15
, 310 (501-2).
Perez-Reyes E
et al.
Molecular characterization of a neuronal low-voltage-activated T-type calcium channel.
Nature,
1998
Feb
26
, 391 (896-900).
Cribbs LL
et al.
Cloning and characterization of alpha1H from human heart, a member of the T-type Ca2+ channel gene family.
Circ. Res.,
1998
Jul
13
, 83 (103-9).
Lee JH
et al.
Cloning and expression of a novel member of the low voltage-activated T-type calcium channel family.
J. Neurosci.,
1999
Mar
15
, 19 (1912-21).
Talley EM
et al.
Differential distribution of three members of a gene family encoding low voltage-activated (T-type) calcium channels.
J. Neurosci.,
1999
Mar
15
, 19 (1895-911).
Kim D
et al.
Thalamic control of visceral nociception mediated by T-type Ca2+ channels.
Science,
2003
Oct
3
, 302 (117-9).
Ikeda H
et al.
Synaptic plasticity in spinal lamina I projection neurons that mediate hyperalgesia.
Science,
2003
Feb
21
, 299 (1237-40).
Shin JB
et al.
A T-type calcium channel required for normal function of a mammalian mechanoreceptor.
Nat. Neurosci.,
2003
Jul
, 6 (724-30).
Kawai F
et al.
Enhancement by T-type Ca2+ currents of odor sensitivity in olfactory receptor cells.
J. Neurosci.,
2001
May
15
, 21 (RC144).
Contributors: Rajnish Ranjan, Michael Schartner
To cite this page: [Contributors] Channelpedia https://channelpedia.epfl.ch/wikipages/87/ , accessed on 2024 Apr 27