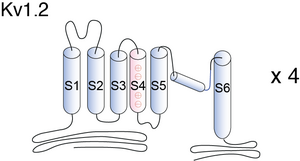
Kv1.2
Description: potassium voltage-gated channel, shaker-related subfamily, member 2 Gene: Kcna2 Alias: Kv1.2, kcna2, BK2, NGK1
Kv1.2, encoded by the gene KCNA2, is a member of the potassium voltage-gated channel subfamily A. Kv1.2 belongs to the delayed rectifier class, members of which allow nerve cells to efficiently repolarize following an action potential. NCBI
Experimental data
Rat Kv1.2 gene in CHO host cells datasheet |
||
|
Click for details 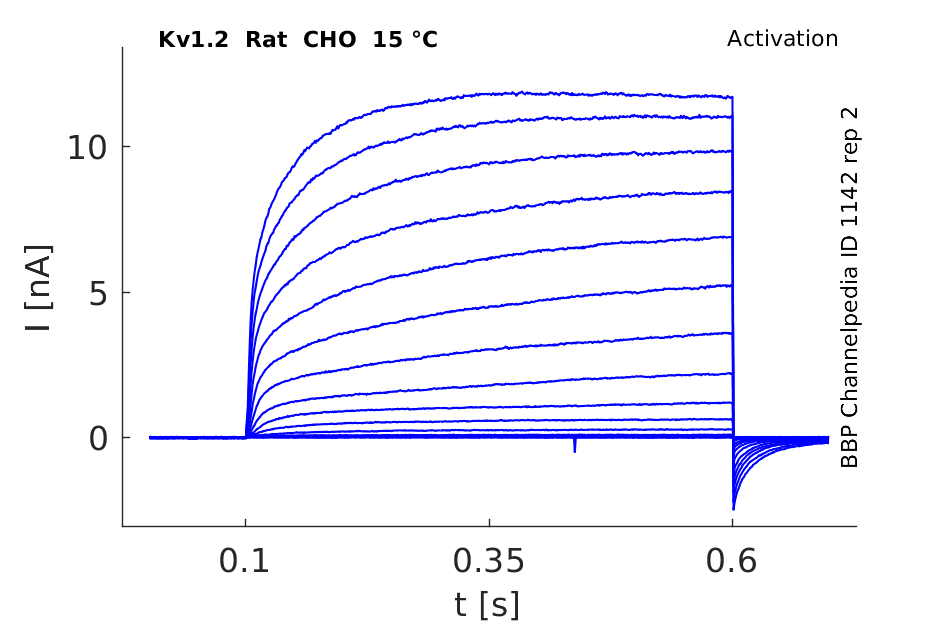
15 °Cshow 116 cells |
Click for details 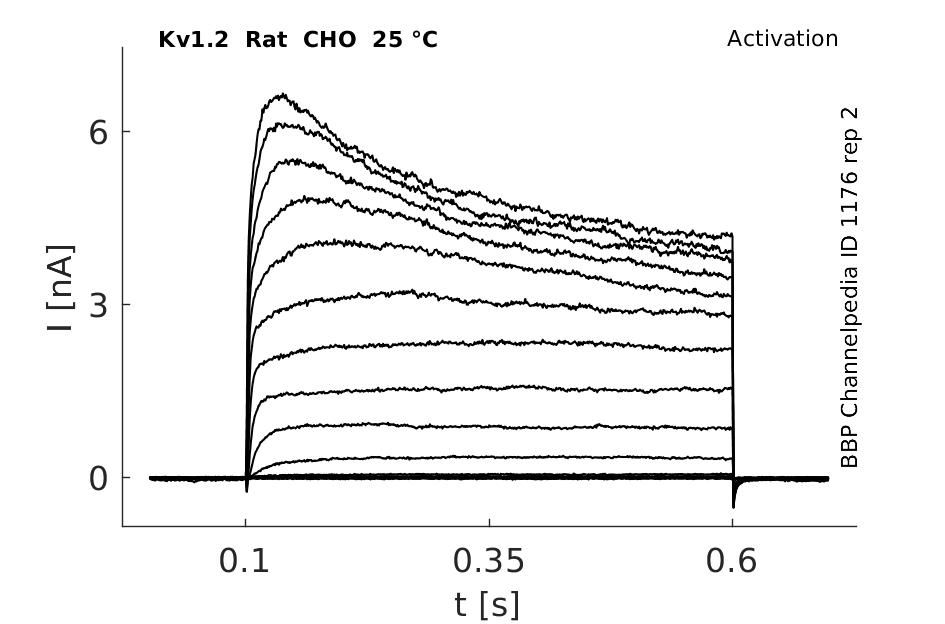
25 °Cshow 161 cells |
Click for details 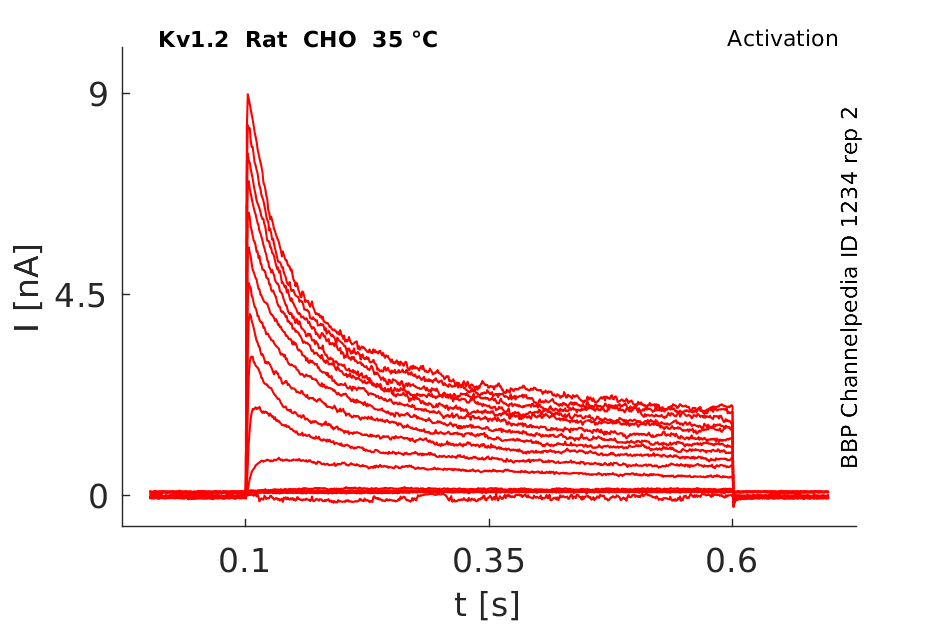
35 °Cshow 57 cells |
The Kcna2 gene is conserved in human, chimpanzee, dog, cow, mouse, chicken, and zebrafish. This gene contains 3 exons (E1 in 5’-UTR, E2 in Coding sequence, E3 in 3’UTR)
The coding region of this gene is intronless, and the gene is clustered with genes KCNA3 and KCNA10 on chromosome 1.
Sequence Conflicts
One sequence conflict described in Rat sequence at location nt 1230-1233 : TTC vs TCT (aa 411 : F (Phe) vs S (ser)).
Isoforms
In Rat, Mouse and hamster only one isoform is described.
*In Human : two different isoforms are described
- Iso a : This variant encodes the longer protein
- Iso b : This variant has multiple differences, compared to variant 1. These differences result in distinct 5' and 3' ends and cause translation termination at a downstream stop codon. The encoded protein (isoform b) is shorter than isoform a (= 356 aa vs 499 aa).
| Species | NCBI accession | Length (nt) | |
|---|---|---|---|
| Human | NM_004974.4 | 11829 | |
| Mouse | NM_008417.5 | 11582 | |
| Rat | NM_012970.4 | 2329 |
Isoforms
Post-Translational Modifications
Visual Representation of Kv1.2 Structure
Methodology for visual representation of structure available here
Crystal Sturcture
The voltage sensors of the Kv1.2 channel are nearly independent domains inside the membrane, that perform mechanical work on the pore through the S4-S5 linker helices, which are positioned to constrict or dilate the S6 inner helices of the pore. Two of the four conserved Arg residues on S4 are on a lipid-facing surface and two are buried in the voltage sensor. [382]. A computer model was built to predict the structure [1365]

Voltage-dependent potassium channels (Kv) are homotetramers composed of four voltage sensors and one pore domain. Because of high-level structural flexibility, the first mammalian Kv structure, Kv1.2 at 2.9 A, has about 37% molecular mass of the transmembrane portion not resolved. In this study, by applying a novel normal-mode-based X-ray crystallographic refinement method to the original diffraction data and structural model, we established the structure of full-length Kv1.2 in its native form [1789]
Block of Kv1.2 Examined
Using both Brownian and molecular dynamics, we replicate many of the salient features of Kv1.2, including the current-voltage-concentration profiles and the binding affinity and binding mechanisms of charybdotoxin, a scorpion venom. We also elucidate how structural differences in the inner vestibule can give rise to significant differences in its permeation characteristics. Current-voltage-concentration profiles are constructed using Brownian dynamics simulations, based on the crystal structure 2A79. The results are compatible with experimental data, showing similar conductance, rectification, and saturation with current. Unlike KcsA, for example, the inner pore of Kv1.2 is mainly hydrophobic and neutral, and to explore the consequences of this, we investigate the effect of mutating neutral proline residues at the mouth of the inner vestibule to charged aspartate residues. We find an increased conductance, less inward rectification, and quicker saturation of the current-voltage profile [1788]
Kv1.2 predicted AlphaFold size
Methodology for AlphaFold size prediction and disclaimer are available here
Single channel Current
18ps
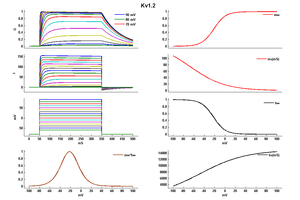
Kv1.2 Kinetics
Kv1.2 channels have half-activation (V 1/2) values ranging from -40mV to +30 mV due to two distinct gating modes, called "slow" and "fast". "Slow" gating (tau around 90ms at 35 mV) was associated with V 1/2 of activation of around 16mV, whereas "fast" gating (tau around 4.5ms at 35 mV) was associated with a V 1/2 of activation of around -19mV. It was possible to switch between gating modes by applying a prepulse, which suggested that channels activate to a single open state along separate "fast" and "slow" activation pathways.[2]

Mouse Kv1.2 expressed in CHO cells

Kv1.2 gating charge in Xenopus oocytes compared to Shaker
Kv1.2 channel closure kinetics were measured in Xenopus oocytes using a tail current protocol. The Kv1.2 gating charge was determined to be near∼25% less than the 13-14 elementary charges in Shaker. Mutation studies of the S4 transmembrane domaine point at a thicker septum separating the aqueous crevices in the voltage-sensing domaine of Kv1.2 as the cause for the lower gating charges [1930]
Human Kv1.2 currents in Xenopus oocytes and the effect of gintonin
Large outward hKv1.2 channel currents were recorded in Xenopus oocytes by applying voltage pulses of 500 ms duration with 10 s intervals and increases of 10 mV from the holding potential of −80 mV, portraying typical delayed rectifier current properties. The outward K+ current was largely diminished in the presence of gintonin.[1605]
Kv1.2 V50 measured in Xenopus oocytes
Conductances were determined in Xenopus oocytes expressing WT or mutated Kv1.2 channels and used to calculate V50 values. WT Kv1.2 channels displayed a V50 of -13.3±1.8 mV. Mutations causing glutamate loops generated the most negative shifts of down to -28.3±1.5 mV. Two residues inserted into loops resulted in a positive shift by up to 55 mV compared to WT Kv1.2.[1923]
Kv1.2 currents recorded in tsa-201 cells and PI(4,5)P2-dependence
Kv1.2 channels were transiently expressed in tsa-201 cells and currents were recorded using the whole-cell technique. Depolar¬izing pulses to 20 mV elicited outward K+ currents. PI(4,5)P2 was depleted by co-expression with PLC-coupled M1 muscarinic recep¬tors (M1R) and receptor activation in Kv1.2 expressing tsa-201 cells. This lead to a decrease of the current amplitude by 27 ± 6% indicating a PI(4,5)P2-dependence of KV1.2 currents.[1925]
Kv1.2 conductance–voltage relationship and positive modulation by sevoflurane
The Kv1.2 conductance–voltage relationship was determined in Xenopus oocytes. Application of the anesthetic sevoflurane shifted the G-V relation to the left and increased Gmax by 13%.[1933]
Prepulse-dependent in Kv1.2 activation measeaured in LM cells
Whole cell patch-clamp recordings were performed on mouse fibroplast LM cells expressing Kv1.2 channels. The results demonstrated that a prepulse-dependent shift in activation is specific to Kv1.2 channels and heteromeric channels containing the Kv1.2 subunit. Prepulse activation kinetics also display a left-shift of V1/2.[1943]
Markov Model Kv1.2
Kv1.2 Hodgkin and Huxley Inactivation

Kv1.2 Hodgkin and Huxley Avtivation

Model Kv1.2 (ID=19)
| Animal | rat | |
| CellType | Oocyte | |
| Age | 0 Days | |
| Temperature | 20.0°C | |
| Reversal | -65.0 mV | |
| Ion | K + | |
| Ligand ion | ||
| Reference | [272] L K Sprunger et. al; Eur. J. Pharmacol. 1996 Oct 31 | |
| mpower | 1.0 | |
| m Inf | 1.0000/(1+ exp((v - -21.0000)/-11.3943)) | |
| m Tau | 150.0000/(1+ exp((v - -67.5600)/34.1479)) | |
| hpower | 1.0 | |
| h Inf | 1.0000/(1+ exp((v - -22.0000)/11.3943)) | |
| h Tau | 15000.0000/(1+ exp((v - -46.5600)/-44.1479)) | |
Kv1.2 subunits can be found in the AISs of different neurons in the neocortex, hippocampus, main olfactory bulb (MOB) and cerebellum.[329]
Expression in Spinal Cord
Kv1.2 had a similar distribution pattern to Kv1.1. In transverse sections of the spinal cord, Kv1.2 was expressed on NF200-positive axons in all white matter tracts with no labelling on glia. In longitudinal sections of noninjured spinal cord, Kv1.2 was shown to have a highly localized paired staining pattern on axons in both the lateral and dorsal columns [1607]
Distribution in Neuron
Kv1.2 is typically concentrated along axons and axon terminals of neurons, as well as at presynaptic sites[1590]
Kv1.2, are present in multiple subcellular locations including cell somata, juxtaparanodal regions of myelinated axons, synaptic terminals, unmyelinated axons, specialized junctions among axons, and proximal dendrites [1601]
The distribution of Kv1.2 (as well as Kv1.4 and Kv3.4) shows considerable temporal variation of axonal localization in hippocampal neurons. This age-dependent expression is thought to link neuronal activity with hippocampal network maturation. [378]
The gene Kcna2 encodes the voltage-gated potassium channel α-subunit Kv1.2, which is abundantly expressed in the large axon terminals of basket cells that make powerful axo-somatic synapses onto Purkinje cells [373]
Distribution in Rat Cerebellum
The potassium channel Kv1.2 α-subunit is expressed in cerebellar Purkinje cell (PC) dendrites where its pharmacological inhibition increases excitability. Kv1.2 is also expressed in cerebellar basket cell (BC) axon terminals, where its blockade increases BC inhibition of PCs. Secretin receptors are also expressed both in PC dendrites and BC axon terminals [1787]
Repetitive Discharge
Currents in striatal medium spiny neurons attributable to Kv1.2 channels activate rapidly, inactivate slowly, and recover from inactivation slowly. In the subthreshold range (ca.-60 mV), these currents accounted for as much as 50% of the depolarization-activated K+ current. Moreover, their rapid activation and relatively slow deactivation suggests that they contribute to spike afterpotentials regulating repetitive discharge. Confirming somato-dendritic Kv1.2 channels to regulate state transitions and repetitive discharge in striatal medium spiny neurons. [294]
Neuropathic Pain
The Kv1.2 subunit may participate in the formation of Kv channel tetramers in most DRG neurons. Interestingly, peripheral nerve injury down-regulated the expression of Kv1.2 in the injured DRG. This down-regulation may be responsible for the nerve injury induced increase in the ectopic discharge activity observed in DRG neurons. Therefore, DRG Kv.1.2 might be a potential target for neuropathic pain treatment [1602]
After peripheral nerve injury the transcription factor, myeloid zinc finger protein 1 (MZF1), binds to the Kcna2 antisense RNA promoter. Transcription of this long non-coding RNA downregulates Kcna2 mRNA. The decrease in Kv1.2 channel expression reduces total K(v)currents and increases excitability of DRG neurons accompanied by symptoms of neuropathic pain [1924]
Similarily, microinjection of recombinant adeno-associated virus 5 expressing full-length MZF1 into the DRG elicited pain hypersensitivities in rats [1938]
Diabetic hyperalgesia
Kv1 channels that are α-DTX-sensitive were shown to be involved in the regulation of the conduction properties of polymodal nociceptive C-fibers, and their dysfunction may be implicated in diabetic hyperalgesia [1934]
Membrane Polarity
Using Kcna2 null mutant mice, we demonstrate a surprising paradox in changes in the membrane properties of SGNs. The resting membrane potential of Kcna2(-/-) SGNs was significantly hyperpolarized compared with that of age-matched wild-type (WT) SGNs [1604]
Type 2 Diabetes
Peripheral nerve hyperexcitability (PNH) is one of the distal peripheral neuropathy phenotypes often present in patients affected by type 2 diabetes mellitus (T2DM). By using pharmacological inhibitors, we demonstrated that the PNH is mediated by the decreased activity of K(v)1-channels. \it was observed that the diabetic condition led to a reduced presence of the K(v)1.2-subunits in juxtaparanodal regions of peripheral nerves in db/db mice and in nerve biopsies from T2DM patients. Together, these observations indicate that the T2DM condition leads to potassium channel-mediated PNH, thus identifying them as a potential drug target to treat some of the DPN related symptoms [1606]
Neurophysiological Role Kv1.2
Throughout the brain, Kv1.2 regulates neurotransmitter release. At the medial nucleus of the trapezoid body, Kv1.2 channels control action potential firing. In the Calyx of Held, presynaptic Kv1.2 channels regulate glutamate release by preventing hyperexcitability in the axon terminals after an action potential invades. Striatal neurons which terminate in the substantia nigra pars reticulata express high levels of presynaptic Kv1.2, which controls GABA release. In the striatum, Kv1.2 can regulate acetylcholine release, and also GABA release from the entorhinal cortex. In the cerebellum, Kv1.2 regulates GABA release at the basket cell: Purkinje cell synapse (Southan and Robertson, 1998b, a, 2000) and excitatory synaptic input to Purkinje cells.
http://www.uvm.edu/~neurogp/pdfs/Williams%20-%20Dissertation%20Proposal.pdf
Role in LTP induction
Induction of LTP in hippocampal CA3 pyramidal cells (PCs) after a conditioning train of 20 action potentials was accompanied by a reduction in the D-type K(+) current, and absent in KCNA null mice [1926] It was further shown that activity-dependent downregulation of Kv1.2 in CA3-PCs facilitates Na channel activation thereby mediating mossy fibres-induced heterosynaptic LTP of perforant path neurotransmission [1941]
Cocaine response
The sigma-1 receptor (Sig-1R) an interorganelle-signaling modulator asscociated with drug-seeking behaviors forms persistent complexes with Kv1.2 channels in response to cocaine application, resulting in upregulation of D-type K(+) currents in the nucleus accumbens (NAc) and neuronal hypoactivity [1922]
Voltage sensitivity
Mutations of rat Kv1.2 targeting the length and composition of the extracellular loop demonstrate the implication of the connection of S4 to S3 in setting the V50 activation in Kv1 channels [1923]
Training and cardial Kv1.2 expression
Training may reverse the pathological expression of the Kv1.2, Kv1.5, and BKCa channels in aortic myocytes of spontaneously hypertensive rats [1927]
Eye blink conditioning (EBC)
The release of EBC-induced secretin from Purkinje cells modulates EBC acquisition in basket cell pinceaus and/or Purkinje cell dendrites by reducing the surface expression of Kv1.2[1929]
AGEs
Advanced glycation end product (AGE) treatment of isolated coronary vascular smooth muscle cells (VSMCs) leads to reduced expression of Kv1.2, whereas in diabetic rats AEG treatment impairs Kv channel-mediated coronary vasodilation [1932]
Ataxia and myoclonic epilepsy
A newly described mutation of KCNA2 c.890C>A, implicating a critical arginine in the S4 voltage-sensing domaine, has been associated with ataxia and myoclonic epilepsy in a patient pointing at a new channelopathy [1936]
Use-dependent activation
Kv1.2 channel subunits are unique in conferring use-dependent activation properties to heteromeric Kv channels [1939] It was further suggested that use-dependent activation is mediated by an extrinsic regulator binding to the channel at the closed state. Mutation studies demonstrated that the residue Thr252 of Kv1.2 seems to be necessary but not sufficient to enable this interaction [1943]
Mutations and epileptic encephalopathies
Mutations in KCNA2 were attested in six patients diagnosed with epileptic encephalopathy pointing at the involvment of Kv1.2 in neurodevelopmental disorders. Depending on the position of the mutation, Kv1.2 currents are either virtually impaired or the channels are permanently open, which allows conclusions about a hyperexcitable or electrically silenced phenotype of KV1.2-expressing neurons [1940]
Electrocytotaxis
Endogenously generated electric fields (EF) were proposed as possible regulators for cell migration. At EF stimulation Kv1.2 was reported to associate with the actin-binding protein cortactin and to re-locate to the cathode-facing membrane. This supports a proposed mechanism of EF-dependent cytotaxis and Kv1.2 channels as important physiological factors in EF-induced cell migration [1942]
Kv1.2-hERG Chimeric Channel
Here, we tested chimaeras of rat Kv1.2 with the hERG channel for function in Xenopus oocytes and for overexpression in Pichia. Chimaera containing the S1-S6 transmembrane region of HERG showed functional and pharmacological properties similar to hERG and could be overexpressed and purified from Pichia. Here, we report successful generation of a Kv1.2 chimaera that contained the S1–S6 transmembrane portion of hERG. It not only exhibited the functional and pharmacological properties of hERG, but could be overexpressed in Pichia pastoris. Co-incidentally, the study also provided important insights into the structure-activity relationships of hERG [1648]
Glycosylation State
Compared with wild Kv1.2, increasing the glycosylation state shifted the V1/2 negatively with a steeper G–V slope, increased activation kinetics with little change in deactivation kinetics or in their voltage-dependence, and decreased the apparent level of C-type inactivation. Decreasing the glycosylation state had essentially the opposite effects and shifted the V1/2 positively with a shallower G–V slope, decreased activation kinetics (and voltagedependence), decreased deactivation kinetics, and increased the apparent level of C type inactivation. Single channel conductance was not affected by the different glycosylation states of Kv1.2.[3]
Bis(7)-tacrine
Rectifier potassium currents in rat DRG neurons were delayed and currents recorded from oocytes expressing K(V)1.2 were suppressed by bis(7)-tacrine, the potency of which was two orders greater than that of tacrine.[4]
Dendrotoxin/Charybdotoxin
Dendrotoxin inhibited channel activation with an IC50 of 8.6 nM at + 35 mV. Charybdotoxin also blocked this K+ channel. While dendrotoxin block was not affected by channel activation, charybdotoxin exhibited additional accumulation of block following activation, which was relieved with a time constant of 0.5 s upon repolarization of the membrane. The deactivation of this channel was accelerated in the presence of charybdotoxin while not significantly affected by dendrotoxin.[272]
GPCRs including mAChR
G protein-coupled receptors (GPCRs), including the m1 muscarinic acetylcholine receptor (mAChR), regulate a tyrosine kinase activity that phosphorylates and suppresses current generated by the Kv1.2 potassium channel [1599]
TEA
TEA (tetraethylammonium) is a classical Kv channel blocker, from the external or internal side of the pore region. For external TEA, neuronal Kv1 channels are either sensitive (IC50=0.3–10 mM; Kv1.1, Kv1.3 and Kv1.6) or insensitive (IC50>100 mM; Kv1.2, Kv1.4, Kv1.5 and Kv1.7). In TEA-sensitive Kv1 channels, all four subunits make an energetic contribution to binding a single TEA molecule at the extracellular mouth, where an aromatic residue in the pore-forming region is required for high sensitivity. TEA sensitivity is mainly due to the presence in Kv1.1 of that critical residue, whereas the Kv1.2 tetrameric channel which has valine (Val381) at the equivalent location is only feebly interrupted by TEA [1603]
VAl381 in-sensitizes Kv1.2 to TEA
In TEA-sensitive Kv1 channels, all four subunits make an energetic contribution to binding a single TEA molecule at the extracellular mouth, where an aromatic residue in the pore-forming region is required for high sensitivity. TEA sensitivity is mainly due to the presence in Kv1.1 of that critical residue, whereas the Kv1.2 tetrameric channel which has valine (Val381) at the equivalent location is only feebly interrupted by TEA [1603]
Kv1.2 interacts with Kv1.4
In vivo and in vitro heteromultimerization of Kv1.2 and Kv1.4 α-subunits underlies the striking and unexpected alterations in the properties of SGNs. The results suggest that heteromeric interactions of Kv1.2 and Kv1.4 dominate the defining features of Kv1 channels in SGNs [1604]
Gintonin
Gintonin is a novel ginseng-derived G protein-coupled lysophosphatidic acid (LPA) receptor ligand. It has been shown that Kv channels can be regulated by Gαq/11 protein-coupled receptor ligands. Gintonin treatment inhibited Kv1.2 channel activity in reversible and concentration-dependent manners. The inhibitory effect of gintonin on Kv1.2 channel activity was blocked by active phospholipase C inhibitor, inositol 1,4,5-triphosphate receptor antagonist, and intracellular Ca(2+) chelator [1605]
Maurotoxin
The 34-residue polypeptide maurotoxin (MTx) isolated from scorpion venoms selectively inhibits the current of the voltage-gated potassium channel Kv1.2 by occluding the ion conduction pathway. Here using molecular dynamics simulation as a docking method, the binding modes of MTx to three closely related channels (Kv1.1, Kv1.2 and Kv1.3) are examined. We show that MTx forms more favorable electrostatic interactions with the outer vestibule of Kv1.2 compared to Kv1.1 and Kv1.3, consistent with the selectivity of MTx for Kv1.2 over Kv1.1 and Kv1.3 observed experimentally.
Phosphatidylinositol-4,5-bisphosphate (PIP2
In experiments using whole-cell methods a PI(4,5)P 2-dependence of KV 1.2 currents in cell culture was demonstrated [1925] Also, Kv1.1/Kv1.2 heteromeric channels in spiral ganglion neurons were positively regulated by PI(4,5)P 2, a possible intrinsic control of rapid spike adaption in the auditory nerve [1914]
Urotoxin
Scorpion peptide venom urotoxin binds with high affinity to hKv1.2 (IC50 of 160 pM),hKv1.1 and hKv1.3 channels (IC50 = 253 nM and 91 nM, respectively)[1889]
Mesomartoxin
Mesomartoxin, a K(v)1.2-selective scorpion toxin was shown to interact with the channel selectivity filter [1931]
Ts6 toxin
Scorpion neurotoxin Ts6 shows high affinity for Kv1.2, Shaker IR and very high affinity to Kv1.3 [1928]
Sevoflurane
Mutation studies indicated that the allosteric modulations of Kv1.2 by sevoflurane are T1-independent. Free-energy calculations suggest that sevoflurane occupies sites near the S4-S5 linker [1933]
Porphyrins
Kv1.1 and Kv1.2 are differentially blocked by porphyrins with different side chains. Porphyrins with 2-4 carbon alkyl ammonium side chains preferentially inhibit Kv1.1 channels, those bearing polyamine side chains Kv1.2 channels [1935]
Gambierol
Cytotoxicity of gambierol and its analogs could not be linked to Kv1.2 channels in a CHO cell electrophysiogical assay and may rather be due to leak currents [1937]
References
Rezazadeh S
et al.
An activation gating switch in Kv1.2 is localized to a threonine residue in the S2-S3 linker.
Biophys. J.,
2007
Dec
15
, 93 (4173-86).
Watanabe I
et al.
The glycosylation state of Kv1.2 potassium channels affects trafficking, gating, and simulated action potentials.
Brain Res.,
2007
May
4
, 1144 (1-18).
Inhibition by bis(7)-tacrine of native delayed rectifier and KV1.2 encoded potassium channels.
Neurosci. Lett.,
2007
Jan
29
, 412 (108-13).
Sprunger LK
et al.
Effects of charybdotoxin on K+ channel (KV1.2) deactivation and inactivation kinetics.
Eur. J. Pharmacol.,
1996
Oct
31
, 314 (357-64).
Shen W
et al.
Kv1.2-containing K+ channels regulate subthreshold excitability of striatal medium spiny neurons.
J. Neurophysiol.,
2004
Mar
, 91 (1337-49).
Lorincz A
et al.
Cell-type-dependent molecular composition of the axon initial segment.
J. Neurosci.,
2008
Dec
31
, 28 (14329-40).
Xie G
et al.
A new Kv1.2 channelopathy underlying cerebellar ataxia.
J. Biol. Chem.,
2010
Oct
15
, 285 (32160-73).
Prüss H
et al.
Age-dependent axonal expression of potassium channel proteins during development in mouse hippocampus.
,
2009
Dec
12
, ().
Long SB
et al.
Crystal structure of a mammalian voltage-dependent Shaker family K+ channel.
Science,
2005
Aug
5
, 309 (897-903).
Sheng M
et al.
Contrasting subcellular localization of the Kv1.2 K+ channel subunit in different neurons of rat brain.
J. Neurosci.,
1994
Apr
, 14 (2408-17).
Klumpp DJ
et al.
The Shaker-like potassium channels of the mouse rod bipolar cell and their contributions to the membrane current.
J. Neurosci.,
1995
Jul
, 15 (5004-13).
Xu H
et al.
Developmental analysis reveals mismatches in the expression of K+ channel alpha subunits and voltage-gated K+ channel currents in rat ventricular myocytes.
J. Gen. Physiol.,
1996
Nov
, 108 (405-19).
Baştuğ T
et al.
Comparative Study of the Energetics of Ion Permeation in Kv1.2 and KcsA Potassium Channels.
Biophys. J.,
2011
Feb
2
, 100 (629-36).
Zhao LL
et al.
Length-dependent regulation of the Kv1.2 channel activation by its C-terminus.
Mol. Membr. Biol.,
2009
Apr
, 26 (186-93).
Coleman SK
et al.
Subunit composition of Kv1 channels in human CNS.
J. Neurochem.,
1999
Aug
, 73 (849-58).
Camacho CJ
Quantitative modeling of currents from a voltage gated ion channel undergoing fast inactivation.
PLoS ONE,
2008
, 3 (e3342).
Yeheskel A
et al.
Independent and cooperative motions of the Kv1.2 channel: voltage sensing and gating.
Biophys. J.,
2010
May
19
, 98 (2179-88).
Małysiak K
et al.
Electrostatic interactions during Kv1.2 N-type inactivation: random-walk simulation.
Eur. Biophys. J.,
2009
Sep
, 38 (1003-12).
Upadhyay SK
et al.
Potassium channel opening: a subtle two-step.
J. Physiol. (Lond.),
2009
Aug
1
, 587 (3851-68).
Khalili-Araghi F
et al.
Dynamics of K+ ion conduction through Kv1.2.
Biophys. J.,
2006
Sep
15
, 91 (L72-4).
Inda MC
et al.
Voltage-gated ion channels in the axon initial segment of human cortical pyramidal cells and their relationship with chandelier cells.
Proc. Natl. Acad. Sci. U.S.A.,
2006
Feb
21
, 103 (2920-5).
Scholle A
et al.
Structural elements determining activation kinetics in Kv2.1.
Recept. Channels,
2000
, 7 (65-75).
Yang H
et al.
A Theoretical Model for Calculating Voltage Sensitivity of Ion Channels and the Application on Kv1.2 Potassium Channel.
Biophys. J.,
2012
Apr
18
, 102 (1815-25).
Chen R
et al.
Structural basis of the selective block of Kv1.2 by maurotoxin from computer simulations.
PLoS ONE,
2012
, 7 (e47253).
Robbins CA
et al.
Kv1.1 and Kv1.2: Similar channels, different seizure models.
Epilepsia,
2012
Jun
, 53 Suppl 1 (134-41).
Tsai W
et al.
Receptor protein tyrosine phosphatase alpha participates in the m1 muscarinic acetylcholine receptor-dependent regulation of Kv1.2 channel activity.
EMBO J.,
1999
Jan
4
, 18 (109-18).
Goodchild SJ
et al.
Basis for allosteric open-state stabilization of voltage-gated potassium channels by intracellular cations.
J. Gen. Physiol.,
2012
Nov
, 140 (495-511).
Wang H
et al.
Localization of Kv1.1 and Kv1.2, two K channel proteins, to synaptic terminals, somata, and dendrites in the mouse brain.
J. Neurosci.,
1994
Aug
, 14 (4588-99).
Fan L
et al.
Impaired neuropathic pain and preserved acute pain in rats overexpressing voltage-gated potassium channel subunit Kv1.2 in primary afferent neurons.
Mol Pain,
2014
, 10 (8).
Al-Sabi A
et al.
Pharmacological characteristics of Kv1.1- and Kv1.2-containing channels are influenced by the stoichiometry and positioning of their α subunits.
Biochem. J.,
2013
Aug
15
, 454 (101-8).
Wang W
et al.
Association of the Kv1 family of K+ channels and their functional blueprint in the properties of auditory neurons as revealed by genetic and functional analyses.
J. Neurophysiol.,
2013
Oct
, 110 (1751-64).
Lee JH
et al.
Activation of lysophosphatidic acid receptor by gintonin inhibits Kv1.2 channel activity: involvement of tyrosine kinase and receptor protein tyrosine phosphatase α.
Neurosci. Lett.,
2013
Aug
26
, 548 (143-8).
Zenker J
et al.
Altered distribution of juxtaparanodal kv1.2 subunits mediates peripheral nerve hyperexcitability in type 2 diabetes mellitus.
J. Neurosci.,
2012
May
30
, 32 (7493-8).
Nashmi R
et al.
Abnormal axonal physiology is associated with altered expression and distribution of Kv1.1 and Kv1.2 K+ channels after chronic spinal cord injury.
Eur. J. Neurosci.,
2000
Feb
, 12 (491-506).
Dhillon MS
et al.
A functional Kv1.2-hERG chimaeric channel expressed in Pichia pastoris.
Sci Rep,
2014
, 4 (4201).
Williams MR
et al.
Cellular mechanisms and behavioral consequences of Kv1.2 regulation in the rat cerebellum.
J. Neurosci.,
2012
Jul
4
, 32 (9228-37).
Gordon D
et al.
Permeation and block of the Kv1.2 channel examined using brownian and molecular dynamics.
Biophys. J.,
2011
Dec
7
, 101 (2671-8).
Chen X
et al.
Structure of the full-length Shaker potassium channel Kv1.2 by normal-mode-based X-ray crystallographic refinement.
Proc. Natl. Acad. Sci. U.S.A.,
2010
Jun
22
, 107 (11352-7).
Luna-Ramírez K
et al.
Structure, molecular modeling, and function of the novel potassium channel blocker urotoxin isolated from the venom of the Australian scorpion Urodacus yaschenkoi.
Mol. Pharmacol.,
2014
Jul
, 86 (28-41).
Smith KE
et al.
Phosphoinositide Modulation of Heteromeric Kv1 Channels Adjusts Output of Spiral Ganglion Neurons from Hearing Mice.
J. Neurosci.,
2015
Aug
12
, 35 (11221-32).
Kourrich S
et al.
Dynamic interaction between sigma-1 receptor and Kv1.2 shapes neuronal and behavioral responses to cocaine.
Cell,
2013
Jan
17
, 152 (236-47).
Sand R
et al.
Fine-tuning of voltage sensitivity of the Kv1.2 potassium channel by interhelix loop dynamics.
J. Biol. Chem.,
2013
Apr
5
, 288 (9686-95).
Zhao X
et al.
A long noncoding RNA contributes to neuropathic pain by silencing Kcna2 in primary afferent neurons.
Nat. Neurosci.,
2013
Aug
, 16 (1024-31).
Kruse M
et al.
The phosphoinositide sensitivity of the KV channel family.
Channels (Austin),
2013
Aug
1
, 7 ().
Hyun JH
et al.
Activity-dependent downregulation of D-type K+ channel subunit Kv1.2 in rat hippocampal CA3 pyramidal neurons.
J. Physiol. (Lond.),
2013
Nov
15
, 591 (5525-40).
Li Z
et al.
Exercise training reverses alterations in Kv and BKCa channel molecular expression in thoracic aorta smooth muscle cells from spontaneously hypertensive rats.
J. Vasc. Res.,
2014
, 51 (447-57).
Cerni FA
et al.
Electrophysiological characterization of Ts6 and Ts7, K⁺ channel toxins isolated through an improved Tityus serrulatus venom purification procedure.
Toxins (Basel),
2014
Mar
, 6 (892-913).
Fuchs JR
et al.
Cerebellar secretin modulates eyeblink classical conditioning.
Learn. Mem.,
2014
Dec
, 21 (668-75).
Ishida IG
et al.
Voltage-dependent gating and gating charge measurements in the Kv1.2 potassium channel.
J. Gen. Physiol.,
2015
Mar
16
, ().
Wang X
et al.
Mesomartoxin, a new K(v)1.2-selective scorpion toxin interacting with the channel selectivity filter.
Biochem. Pharmacol.,
2015
Jan
15
, 93 (232-9).
Su W
et al.
Advanced Glycation End Products Impair Voltage-Gated K+ Channels-Mediated Coronary Vasodilation in Diabetic Rats.
PLoS ONE,
2015
, 10 (e0142865).
Liang Q
et al.
Positive Allosteric Modulation of Kv Channels by Sevoflurane: Insights into the Structural Basis of Inhaled Anesthetic Action.
PLoS ONE,
2015
, 10 (e0143363).
Wang XC
et al.
α-dendrotoxin sensitive Kv1 channels contribute to conduction failure of polymodal nociceptive C-fibers from rat coccygeal nerve.
J. Neurophysiol.,
2015
Nov
25
, (jn.00786.2014).
Daly D
et al.
Porphyrin derivatives as potent and selective blockers of neuronal Kv1 channels.
Chem. Commun. (Camb.),
2015
Jan
21
, 51 (1066-9).
Pena SD
et al.
Ataxia and myoclonic epilepsy due to a heterozygous new mutation in KCNA2: proposal for a new channelopathy.
Clin. Genet.,
2015
Feb
, 87 (e1-3).
Konoki K
et al.
Evaluation of gambierol and its analogs for their inhibition of human Kv1.2 and cytotoxicity.
Bioorg. Med. Chem. Lett.,
2015
Feb
1
, 25 (514-8).
Li Z
et al.
Dorsal root ganglion myeloid zinc finger protein 1 contributes to neuropathic pain after peripheral nerve trauma.
Pain,
2015
Apr
, 156 (711-21).
Baronas VA
et al.
Use-dependent activation of neuronal kv1.2 channel complexes.
J. Neurosci.,
2015
Feb
25
, 35 (3515-24).
Syrbe S
et al.
De novo loss- or gain-of-function mutations in KCNA2 cause epileptic encephalopathy.
Nat. Genet.,
2015
Apr
, 47 (393-9).
Hyun JH
et al.
Kv1.2 mediates heterosynaptic modulation of direct cortical synaptic inputs in CA3 pyramidal cells.
J. Physiol. (Lond.),
2015
Aug
15
, 593 (3617-43).
Zhang G
et al.
The Role of Kv1.2 Channel in Electrotaxis Cell Migration.
J. Cell. Physiol.,
2015
Nov
18
, ().
Baronas VA
et al.
Determinants of frequency-dependent regulation of Kv1.2-containing potassium channels.
Channels (Austin),
2015
Dec
8
, (0).
Contributors: Rajnish Ranjan, Michael Schartner, Erika Borcel, Nitin Khanna
To cite this page: [Contributors] Channelpedia https://channelpedia.epfl.ch/wikipages/2/ , accessed on 2024 Apr 27