Kv6.3
Description: potassium voltage-gated channel, subfamily G, member 3 Gene: Kcng3 Alias: Kv6.3, kcng3, kv10.1
Kv6.3, encoded by the gene KCNG3, is a member is a gamma subunit of the voltage-gated potassium channel, subfamily G. Kv6.3 is thought to be a delayed-rectifier type channels that may contribute to cardiac action potential repolarization. NCBI
Experimental data
Rat Kv6.3 gene in CHO host cells datasheet |
||
|
Click for details 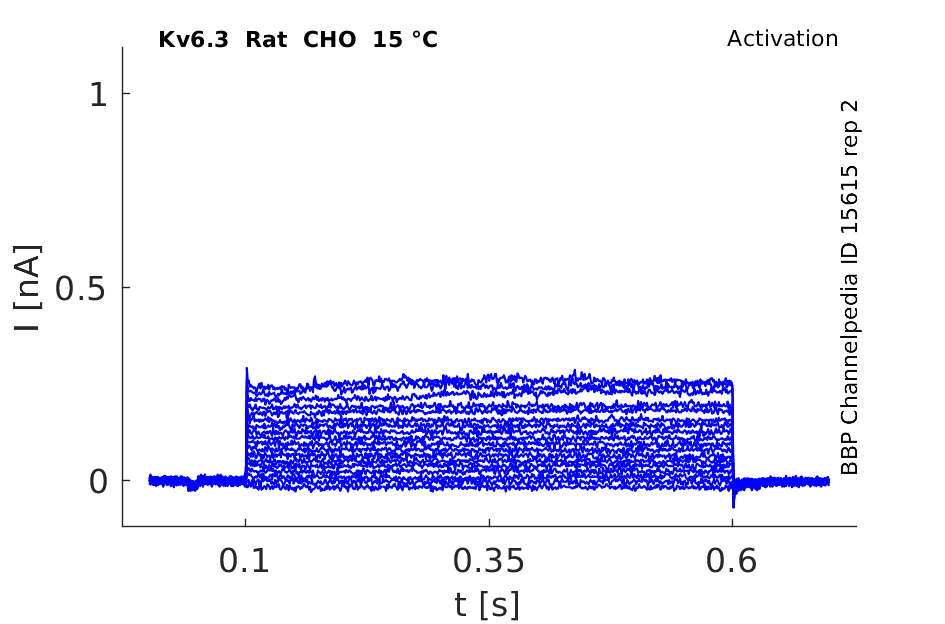
15 °Cshow 68 cells |
Click for details 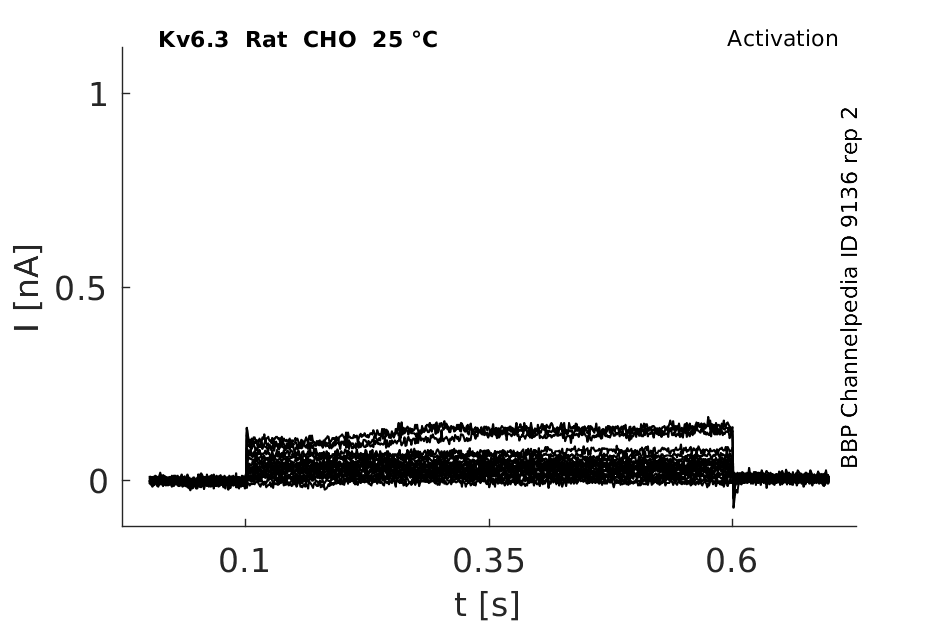
25 °Cshow 61 cells |
Click for details 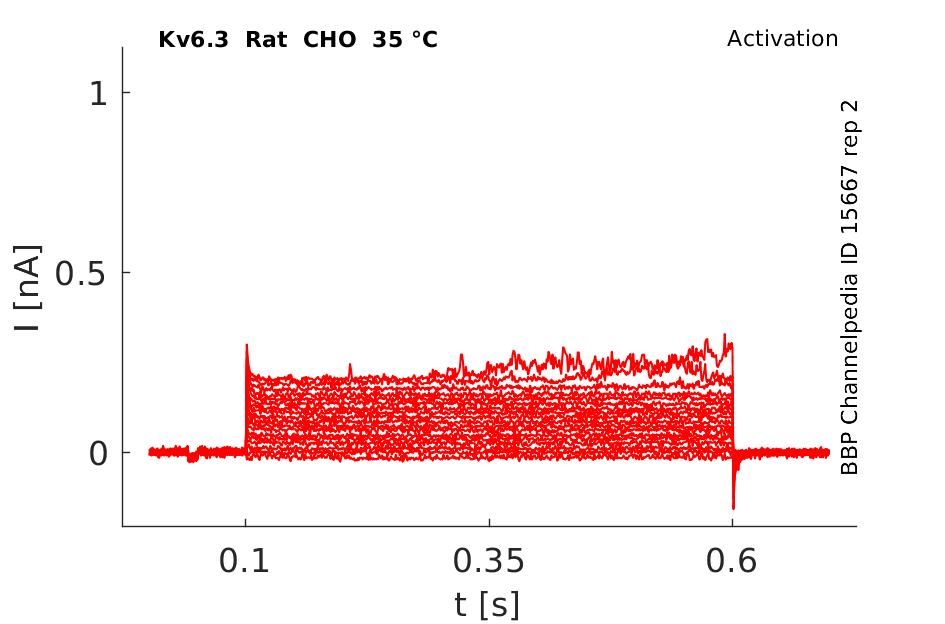
35 °Cshow 87 cells |
Gene
Transcript
| Species | NCBI accession | Length (nt) | |
|---|---|---|---|
| Human | NM_133329.6 | 3709 | |
| Mouse | NM_153512.1 | 3356 | |
| Rat | NM_133426.2 | 1586 |
Protein Isoforms
Isoforms
Post-Translational Modifications
Visual Representation of Kv6.3 Structure
Methodology for visual representation of structure available here
Kv6.3 predicted AlphaFold size
Methodology for AlphaFold size prediction and disclaimer are available here
Interaction of all Kv6.3 subunit on Kv2.1
Whole-cell current recordings and subcellular localization of Kv2.1GFP, Kv6.3GFP and the coexpression Currents were evoked by stepping from −80 mV to +70 mV, 500 ms in duration, followed by a repolarizing pulse at −30 mV, 1 s in duration. Co-expression of Kv2.1 with Kv6.3GFP resulted in outward currents and green plasma membrane staining. This demonstrates that Kv2.1 was able to rescue the Kv6.3GFP subunits out of the ER [1708]
The profound effects of Kv6.3 on Kv2.1 gating properties suggest an important role for these heterotetramers: the latter would be inactivated at potentials close to resting potential (V½ for inactivation is −56 mV) in contrast to the homotetrameric Kv2.1 channels (V½ = −16 mV). Because both subunits are expressed in the brain functional heterotetramers could exist. Previous studies on the sustained delayed rectifier component of hippocampal neurons showed properties that are comparable with those of Kv2.1 and Kv6.3 heteromultimers. At −5 mV the two time constants for activation for the current in those neurons were 53 ms and 190 ms, which is comparable with heterotetrameric channels of Kv2.1 and Kv6.3 (Table 1). In addition, the midpoint of inactivation was more negative (−96 mV), which is at least closer to −56 mV for Kv2.1 and Kv6.3 compared with −16 mV for Kv2.1 alone [648]
Kv6.3 regulates Kv current amplitude and kinetics observed in vascular smooth muscle cells, suggesting that the remodelling of Kv2 current could be an important determinant of the hypertensive phenotype in resistance arteries. [662], [663]
The voltage-activated potassium channel subunits Kv2.1 and Kv2.2 are capable of heteromeric assembly with members of the Kv6 subfamily, which generates channels with different biophysical properties compared with homomeric Kv2.1 channels [661], [675], [676], [648], [398]. For the influence of Kv6.3 on Kv2.1, see [664].
In Kv6.x channels the histidine residue of the zinc ion-coordinating C3H1 motif of Kv2.1 is replaced by arginine or valine. Using a yeast two-hybrid assay, we found that substitution of the corresponding histidine 105 in Kv2.1 by valine (H105V) or arginine (H105R) disrupted the interaction of the T1 domain of Kv2.1 with the T1 domains of both Kv6.3 and Kv6.4, whereas interaction of the T1 domain of Kv2.1 with itself was unaffected by this mutation [664]
Kv2.1
The voltage-activated K(+) channel subunit Kv2.1 can form heterotetramers with members of the Kv6 subfamily, generating channels with biophysical properties different from homomeric Kv2.1 channels. The N-terminal tetramerization domain (T1) has been shown previously to play a role in Kv channel assembly [644]
References
Kramer JW
et al.
Modulation of potassium channel gating by coexpression of Kv2.1 with regulatory Kv5.1 or Kv6.1 alpha-subunits.
Am. J. Physiol.,
1998
Jun
, 274 (C1501-10).
Coetzee WA
et al.
Molecular diversity of K+ channels.
Ann. N. Y. Acad. Sci.,
1999
Apr
30
, 868 (233-85).
Li M
et al.
Specification of subunit assembly by the hydrophilic amino-terminal domain of the Shaker potassium channel.
Science,
1992
Aug
28
, 257 (1225-30).
Lu J
et al.
T1-T1 interactions occur in ER membranes while nascent Kv peptides are still attached to ribosomes.
Biochemistry,
2001
Sep
18
, 40 (10934-46).
Jan LY
et al.
Voltage-gated and inwardly rectifying potassium channels.
J. Physiol. (Lond.),
1997
Dec
1
, 505 ( Pt 2) (267-82).
Ottschytsch N
et al.
Obligatory heterotetramerization of three previously uncharacterized Kv channel alpha-subunits identified in the human genome.
Proc. Natl. Acad. Sci. U.S.A.,
2002
Jun
11
, 99 (7986-91).
Xu J
et al.
Assembly of voltage-gated potassium channels. Conserved hydrophilic motifs determine subfamily-specific interactions between the alpha-subunits.
J. Biol. Chem.,
1995
Oct
20
, 270 (24761-8).
Zhu XR
et al.
Structural and functional characterization of Kv6.2 a new gamma-subunit of voltage-gated potassium channel.
Recept. Channels,
1999
, 6 (337-50).
Moreno-Domínguez A
et al.
De novo expression of Kv6.3 contributes to changes in vascular smooth muscle cell excitability in a hypertensive mice strain.
J. Physiol. (Lond.),
2009
Feb
1
, 587 (625-40).
Cox RH
et al.
New expression profiles of voltage-gated ion channels in arteries exposed to high blood pressure.
,
2002
, 9 (243-57).
Mederos Y Schnitzler M
et al.
Mutation of histidine 105 in the T1 domain of the potassium channel Kv2.1 disrupts heteromerization with Kv6.3 and Kv6.4.
J. Biol. Chem.,
2009
Feb
13
, 284 (4695-704).
Hille B
Ionic selectivity of Na and K channels of nerve membranes.
Membranes,
1975
, 3 (255-323).
Barry DM
et al.
Myocardial potassium channels: electrophysiological and molecular diversity.
Annu. Rev. Physiol.,
1996
, 58 (363-94).
Robinson JM
et al.
Coupled tertiary folding and oligomerization of the T1 domain of Kv channels.
Neuron,
2005
Jan
20
, 45 (223-32).
Bixby KA
et al.
Zn2+-binding and molecular determinants of tetramerization in voltage-gated K+ channels.
Nat. Struct. Biol.,
1999
Jan
, 6 (38-43).
Jahng AW
et al.
Zinc mediates assembly of the T1 domain of the voltage-gated K channel 4.2.
J. Biol. Chem.,
2002
Dec
6
, 277 (47885-90).
Strang C
et al.
The role of Zn2+ in Shal voltage-gated potassium channel formation.
J. Biol. Chem.,
2003
Aug
15
, 278 (31361-71).
Tu L
et al.
Voltage-gated K+ channels contain multiple intersubunit association sites.
J. Biol. Chem.,
1996
Aug
2
, 271 (18904-11).
Vega-Saenz de Miera EC
Modification of Kv2.1 K+ currents by the silent Kv10 subunits.
Brain Res. Mol. Brain Res.,
2004
Apr
7
, 123 (91-103).
Sano Y
et al.
Molecular cloning and characterization of Kv6.3, a novel modulatory subunit for voltage-gated K(+) channel Kv2.1.
FEBS Lett.,
2002
Feb
13
, 512 (230-4).
Ottschytsch N
et al.
Domain analysis of Kv6.3, an electrically silent channel.
J. Physiol. (Lond.),
2005
Nov
1
, 568 (737-47).
Contributors: Rajnish Ranjan, Michael Schartner
To cite this page: [Contributors] Channelpedia https://channelpedia.epfl.ch/wikipages/21/ , accessed on 2024 May 04